Learning Outcomes
i. Students will be able to explain the enhanced reactivity of phenol compared to benzene due to the electron-donating hydroxyl group.
ii. Students will understand the electrophilic aromatic substitution reactions of phenol, including halogenation, nitration, and sulfonation.
iii. Students will describe the reaction of phenol with sodium metal to form phenoxide salts and their applications.
iv. Students will explain the oxidation of phenol to form various products, including benzoquinone and hydroquinone.
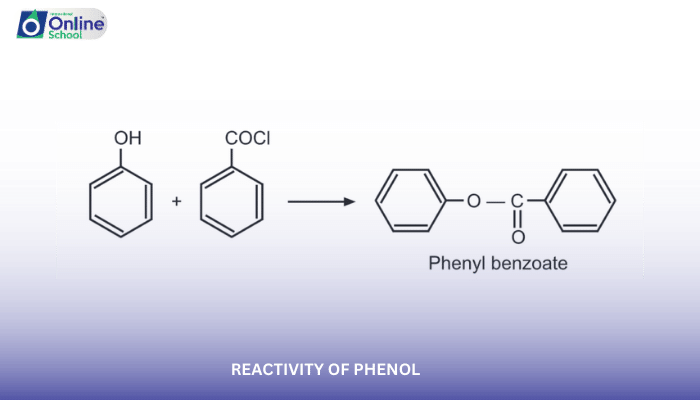
Introduction
Phenol, a versatile organic compound characterized by a hydroxyl group (-OH) attached to a benzene ring, exhibits a unique reactivity pattern due to the electron-donating nature of the hydroxyl group. This electron-rich environment makes phenol more reactive than benzene towards electrophilic aromatic substitution reactions.
i. Electrophilic Aromatic Substitution Reactions of Phenol
Halogenation: Phenol readily undergoes halogenation with halogens like bromine and chlorine under acidic conditions. The electron-donating hydroxyl group activates the benzene ring, making it more susceptible to electrophilic attack.
Nitration: Phenol reacts with nitric acid in the presence of sulfuric acid to form nitrophenols. The hydroxyl group directs the nitration to the ortho and para positions of the benzene ring.
Sulfonation: Phenol reacts with concentrated sulfuric acid to form phenolsulfonic acids. The sulfonation occurs at the para position of the benzene ring.
ii. Reaction of Phenol with Sodium Metal
Phenol reacts with sodium metal in a vigorous reaction to form sodium phenoxide, a salt-like compound. This reaction demonstrates the acidic nature of phenol, as it can release a proton to form the phenoxide ion.
C6H5OH + 2Na → C6H5ONa + H2
iii. Applications of Sodium Phenoxide
Production of dyes: Sodium phenoxide is used as a precursor for the synthesis of azo dyes, widely used in the textile industry.
Synthesis of pharmaceuticals: Sodium phenoxide is used in the production of various pharmaceuticals, such as phenobarbital and salicylaldehyde.
iv. Oxidation of Phenol
Phenol can undergo oxidation reactions under different conditions, leading to the formation of various products.
Benzoquinone: Oxidation of phenol with strong oxidizing agents like potassium dichromate in sulfuric acid produces benzoquinone, a cyclic compound with two carbonyl groups.
Hydroquinone: Oxidation of phenol with milder oxidizing agents like hydrogen peroxide in the presence of a catalyst produces hydroquinone, a benzene derivative with two hydroxyl groups in the ortho positions.
The reactivity of phenol is significantly influenced by the electron-donating hydroxyl group, making it more prone to electrophilic aromatic substitution reactions compared to benzene. The enhanced reactivity of phenol allows for diverse synthetic transformations, leading to various valuable compounds with applications in the chemical industry, pharmaceuticals, and dye production. Understanding the reactivity of phenol is crucial for comprehending its versatility and potential applications.